
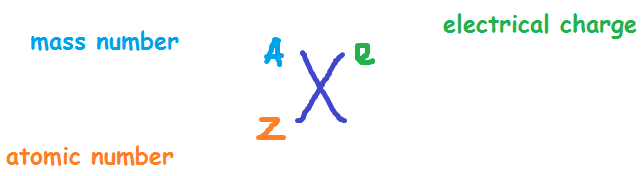
That means there is one more proton than electron. This number may be positive or negative (you could have a 3- there, instead of a 3+, for example). This may be blank - just think of a blank spot meaning "0." If it is not blank, the number there is how many more protons than electrons that the atom has.
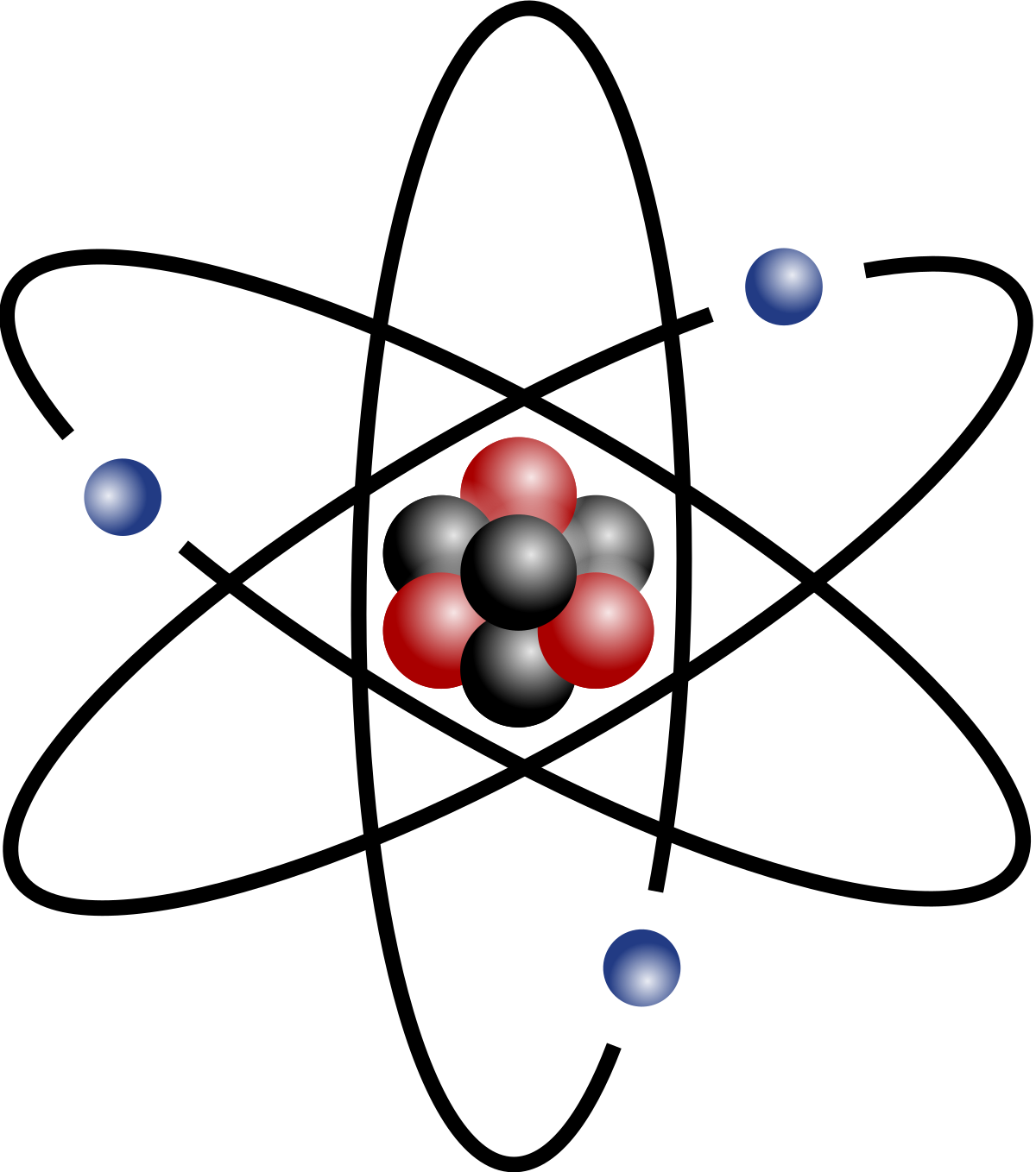
The upper right corner has to do with charge and number of electrons. We see that our atom has 2 protons, so we just subtract: 4 - 2 = 2 neutrons. Use the top left to find the number of neutrons.
#ATOMIC SYMBOL PLUS#
This number corresponds to the number of protons plus the number of neutrons. Now look at the upper left corner, where the "4" is.
This is the atomic number, or the number of protons. Let's start with the lower left corner, where the "2" is. The square has four corners, and sometimes there will be numbers in those corners, like:Įach of these spots may have a number that tells you something: Now imagine a little square around the He. Imagine the atomic symbol of an element, say Helium, He. If the atom is neutral (not an ion), then there are the same number of electrons as protons. That number is the "atomic number" and is equal to the number of protons in the atom. If you're asking this question, I'm sure that you have access to a periodic table of the elements somewhere (if not, there's one at that you can look at) If you look on the periodic table, you will see that there is a number by each element.


 0 kommentar(er)
0 kommentar(er)
